- Phosphorus Valence Electrons
- How Many Phosphorus Valence Electrons
- Phosphorus Valence Electrons Number
- Phosphorus Valence Electrons And Ion
- Phosphorus is an element which is part of Group 15A neutral Phosphorus Atom has five valence electrons. These are contained in the third energy level of the atom. Due to the number of valence electrons, Phosphorus is capable of forming three bonds with other elements. The Valence Electrons are found in different types of orbitals.
- Nitrogen atoms can therefore hold a maximum of eight valence electrons. Phosphorus, however, has empty 3d atomic orbitals that can be used to expand the valence shell to hold 10 or more electrons. Thus, phosphorus can react with fluorine to form both PF 3 and PF 5.
Phosphorus always has 5 valence electrons as spoken. To your second question, this is the way all elements are configured, by adding to the lighter noble gas.
Our editors will review what you’ve submitted and determine whether to revise the article.
Join Britannica's Publishing Partner Program and our community of experts to gain a global audience for your work!Phosphorus (P), nonmetallic chemical element of the nitrogen family (Group 15 [Va] of the periodic table) that at room temperature is a colourless, semitransparent, soft, waxy solid that glows in the dark.
| atomic number | 15 |
|---|---|
| atomic weight | 30.9738 |
| melting point (white) | 44.1 °C (111.4 °F) |
| boiling point (white) | 280 °C (536 °F) |
| density (white) | 1.82 gram/cm3 at 20 °C (68 °F) |
| oxidation states | −3, +3, +5 |
| electron configuration | 1s22s22p63s23p3 |
History
Arabian alchemists of the 12th century may have isolated elemental phosphorus by accident, but the records are unclear. Phosphorus appears to have been discovered in 1669 by Hennig Brand, a German merchant whose hobby was alchemy. Brand allowed 50 buckets of urine to stand until they putrified and “bred worms.” He then boiled the urine down to a paste and heated it with sand, thereby distilling elemental phosphorus from the mixture. Brand reported his discovery in a letter to Gottfried Wilhelm Leibniz, and, thereafter, demonstrations of this element and its ability to glow in the dark, or “phosphoresce,” excited public interest. Phosphorus, however, remained a chemical curiosity until about a century later when it proved to be a component of bones. Digestion of bones with nitric or sulfuric acid formed phosphoric acid, from which phosphorus could be distilled by heating with charcoal. In the late 1800s James Burgess Readman of Edinburgh developed an electric furnace method for producing the element from phosphate rock, which is essentially the method employed today.
Occurrence and distribution
Phosphorus is a very widely distributed element—12th most abundant in crustEarth’s , to which it contributes about 0.10 weight percent. Its cosmic abundance is about one atom per 100 atoms of silicon, the standard. Its high chemical reactivity assures that it does not occur in the free state (except in a few meteorites). Phosphorus always occurs as the phosphateion. The principal combined forms in nature are the phosphate salts. About 550 different minerals have been found to contain phosphorus, but, of these, the principal source of phosphorus is the apatite series in which calcium ions exist along with phosphate ions and variable amounts of fluoride, chloride, or hydroxide ions, according to the formula [Ca10(PO4)6(F, Cl, or OH)2]. Other important phosphorus-bearing minerals are wavellite and vivianite. Commonly, such metal atoms as magnesium, manganese, strontium, and lead substitute for calcium in the mineral, and silicate, sulfate, vanadate, and similar anions substitute for phosphate ions. Very large sedimentary deposits of fluoroapatite are found in many parts of Earth. The phosphate of bone and tooth enamel is hydroxyapatite. (The principle of lessening tooth decay by fluoridation depends upon the conversion of hydroxyapatite to the harder, more decay-resistant, fluoroapatite.)
The chief commercial source is phosphorite, or phosphate rock, an impure massive form of carbonate-bearing apatite. Estimates of the total phosphate rock in Earth’s crust average about 65,000,000,000 tons, of which Morocco and Western Sahara contain about 80 percent. This estimate includes only ore that is sufficiently rich in phosphate for conversion to useful products by present methods. Vast quantities of material lower in phosphorus content also exist.
The only naturally occurring isotope of phosphorus is that of mass 31. The other isotopes from mass 24 to mass 46 have been synthesized by appropriate nuclear reactions. All of these are radioactive with relatively short half-lives. The isotope of mass 32 has a half-life of 14.268 days and has proven extremely useful in tracer studies involving the absorption and movement of phosphorus in living organisms.
Commercial production and uses
The principal technique for converting phosphate rock to usable materials involves acidulation of the crushed rock—with either sulfuric or phosphoric acids—to form crude calciumhydrogen phosphates that, being water-soluble, are valuable additions to fertilizer. Most of the output is burned to phosphoric anhydride and subsequently treated with water to form phosphoric acid, H3PO4. About 95 percent of the phosphate rock mined in the United States is used to make fertilizer or food supplements for animals. Concerns have arisen about phosphorus use, however. Most of the phosphorus is wasted on its journey from mining to being eaten by humans, and the wasted phosphorus ends up in waterways where it can cause algal blooms. Another concern is that increased phosphorus usage will deplete the nonrenewable supply of phosphate rock.
Only about 5 percent of the phosphorus consumed per year in the United States is used in the elemental form. Pyrotechnic applications of the element include tracers, incendiaries, fireworks, and matches. Some is used as an alloying agent, some is used to kill rodents, and the rest is employed in chemical synthesis. A large amount is converted to sulfides used in matches and in the manufacture of insecticides and oil additives. Most of the remainder is converted to halides or oxides for subsequent use in synthesizing organic phosphorus compounds.
- key people
- related topics
Learning Objectives
- Describe how electrons are grouped within atoms.
Although we have discussed the general arrangement of subatomic particles in atoms, we have said little about how electrons occupy the space about the nucleus. Do they move around the nucleus at random, or do they exist in some ordered arrangement?
The modern theory of electron behavior is called quantum mechanics. It makes the following statements about electrons in atoms:
- Electrons in atoms can have only certain specific energies. We say that the energies of the electrons are quantized.
- Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n). Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. Shells do not have specific, fixed distances from the nucleus, but an electron in a higher-energy shell will spend more time farther from the nucleus than does an electron in a lower-energy shell.
- Shells are further divided into subsets of electrons called subshells. The first shell has only one subshell, the second shell has two subshells, the third shell has three subshells, and so on. The subshells of each shell are labeled, in order, with the letters s, p, d, and f. Thus, the first shell has only a single s subshell (called 1s), the second shell has 2s and 2p subshells, the third shell has 3s, 3p, and 3dand so forth.
| Shell | Number of Subshells | Names of Subshells |
|---|---|---|
| 1 | 1 | 1s |
| 2 | 2 | 2s and 2p |
| 3 | 3 | 3s, 3p and 3d |
| 4 | 4 | 4s, 4p, 4d and 4f |
- Different subshells hold a different maximum number of electrons. Any s subshell can hold up to 2 electrons; p, 6; d, 10; and f, 14.
| Subshell | Maximum Number of Electrons |
|---|---|
| s | 2 |
| p | 6 |
| d | 10 |
| f | 14 |
It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that.
We use numbers to indicate which shell an electron is in. As shown in Table (PageIndex{1}), the first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. This first shell has only one subshell, which is labeled 1s and can hold a maximum of 2 electrons. We combine the shell and subshell labels when referring to the organization of electrons about a nucleus and use a superscript to indicate how many electrons are in a subshell. Thus, because a hydrogen atom has its single electron in the s subshell of the first shell, we use 1s1 to describe the electronic structure of hydrogen. This structure is called an electron configuration. Electron configurations are shorthand descriptions of the arrangements of electrons in atoms. The electron configuration of a hydrogen atom is spoken out loud as “one-ess-one.”
Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s2(spoken as “one-ess-two”).
The 1s subshell cannot hold 3 electrons (because an s subshell can hold a maximum of 2 electrons), so the electron configuration for a lithium atom cannot be 1s3. Two of the lithium electrons can fit into the 1s subshell, but the third electron must go into the second shell. The second shell has two subshells, s and p, which fill with electrons in that order. The 2s subshell holds a maximum of 2 electrons, and the 2p subshell holds a maximum of 6 electrons. Because lithium’s final electron goes into the 2s subshell, we write the electron configuration of a lithium atom as 1s22s1. The shell diagram for a lithium atom is shown below. The shell closest to the nucleus (first shell) has 2 dots representing the 2 electrons in 1s, while the outermost shell (2s) has 1 electron.
The next largest atom, beryllium, has 4 electrons, so its electron configuration is 1s22s2. Now that the 2s subshell is filled, electrons in larger atoms start filling the 2p subshell. Thus, the electron configurations for the next six atoms are as follows:
- B: 1s22s22p1
- C: 1s22s22p2
- N: 1s22s22p3
- O: 1s22s22p4
- F: 1s22s22p5
- Ne: 1s22s22p6
With neon, the 2p subshell is completely filled. Because the second shell has only two subshells, atoms with more electrons now must begin the third shell. The third shell has three subshells, labeled s, p, and d. The d subshell can hold a maximum of 10 electrons. The first two subshells of the third shell are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1s22s22p63s23p1. However, a curious thing happens after the 3p subshell is filled: the 4s subshell begins to fill before the 3d subshell does. In fact, the exact ordering of subshells becomes more complicated at this point (after argon, with its 18 electrons), so we will not consider the electron configurations of larger atoms. A fourth subshell, the f subshell, is needed to complete the electron configurations for all elements. An f subshell can hold up to 14 electrons.
Electron filling always starts with 1s, the subshell closest to the nucleus. Next is 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, etc., shown in the electron shell filling order diagram in Figure (PageIndex{2}). Follow each arrow in order from top to bottom. The subshells you reach along each arrow give the ordering of filling of subshells in larger atoms.
Example (PageIndex{1}): Electronic Configuration of Phosphorus Atoms
Using Figure (PageIndex{2}) as your guide, write the electron configuration of a neutral phosphorus atom. The atomic number of P is 15.
Solution
A neutral phosphorus atom has 15 electrons. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. That leaves 5 electrons. Of those 5 electrons, 2 can go into the 3s subshell, and the remaining 3 electrons can go into the 3p subshell. Thus, the electron configuration of neutral phosphorus atoms is 1s22s22p63s23p3.
Exercise (PageIndex{1}): Electronic Configuration of Chlorine Atoms
Using Figure (PageIndex{2}) as your guide, write the electron configuration of a neutral chlorine atom. The atomic number of Cl is 17.
A neutral chlorine atom has 17 electrons. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. That leaves 7 electrons. Of those 7 electrons, 2 can go into the 3s subshell, and the remaining 5 electrons can go into the 3p subshell. Thus, the electron configuration of neutral chlorine atoms is 1s22s22p63s23p5.
Since the arrangement of the periodic table is based on the electron configurations, Figure (PageIndex{3}) provides an alternative method for determining the electron configuration. The filling order simply begins at the top left, with hydrogen (Z=1) and includes each subshell as you proceed in increasing atomic number (Z) order.
For example, the first row (Period 1) contains H and He only, because only two electrons are required to fill the 1s subshell. The second row s-block, contains only two elements, Li and Be, to fill the 2s subshell. This is followed by the second row p-block, containing 6 elements (B through Ne) since six electrons are required to fill the 2p subshell. The third row is similar to the second row elements. Two electrons are needed (Na and Mg) to fill the 3s subshell and six electrons are required (Al through Ar) to complete the 3p subshell. After filling the 3p block up to Ar, we see the next subshell will be 4s (K, Ca), followed by the 3d subshell, which are filled by ten electrons (Sc through Zn). The 4p subshell is filled next by six electrons (Ga through Kr). As you can see, the periodic table shown in Figure (PageIndex{3}) provides a simple way to remember the order of filling the subshells in determining the electron configuration. The order of filling subshells is the same: 1s,2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, etc.
Example (PageIndex{2}): Aluminum
Using Figure (PageIndex{3}) as your guide, write the electron configuration of neutral aluminum atom. The atomic number of Al is 13.
Solution
Aluminum has 13 electrons.
Start at Period 1 of the periodic table, Figure (PageIndex{3}). Place two electrons in the 1s subshell (1s2).
Proceed to Period 2 (left to right direction). Place the next two electrons in the 2s subshell (2s2) and the next six electrons in the 2p subshell (2p6).
Proceed to Period 3 (left to right direction). Place the next two electrons in the 3s subshell (3s2) and the last one electron in the 3p subshell (3p1).
The electron configuration of Aluminum is 1s22s22p63s23p1
Phosphorus Valence Electrons
Exercise (PageIndex{2})
Using Figure (PageIndex{3}) as your guide, write the electron configuration of the atom that has 20 electrons
Start at Period 1 of Figure (PageIndex{3}). Place two electrons in the 1s subshell (1s2).
Proceed to Period 2 (left to right direction). Place the next two electrons in the 2s subshell (2s2) and the next six electrons in the 2p subshell (2p6).
Proceed to Period 3 (left to right direction). Place the next two electrons in the 3s subshell (3s2) and the next six electron in the 3p subshell (3p6).
Proceed to Period 4. Place the remaining two electrons in the 4s subshell (4s2).
The electron configuration is 1s22s22p63s23p64s2
Valence Electrons
In the study of chemical reactivity, we will find that the electrons in the outermost principal energy level are very important and so they are given a special name. Valence electrons are the electrons in the highest occupied principal energy level of an atom.
In the second period elements, the two electrons in the (1s) sublevel are called inner-shell electrons and are not involved directly in the element's reactivity or in the formation of compounds. Lithium has a single electron in the second principal energy level and so we say that lithium has one valence electron. Beryllium has two valence electrons. How many valence electrons does boron have? You must recognize that the second principal energy level consists of both the (2s) and the (2p) sublevels and so the answer is three. In fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. Neon, with its configuration ending in (2s^2 2p^6), has eight valence electrons.
The alkali metal sodium (atomic number 11) has one more electron than the neon atom. This electron must go into the lowest-energy subshell available, the 3s orbital, giving a 1s22s22p63s1 configuration. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell orbitals are called core electrons ( Figure PageIndex4). Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core electron configuration, along with the valence electrons in a condensed format. For our sodium example, the symbol [Ne] represents core electrons, (1s22s22p6) and our abbreviated or condensed configuration is [Ne]3s1.
Similarly, the abbreviated configuration of lithium can be represented as [He]2s1, where [He] represents the configuration of the helium atom, which is identical to that of the filled inner shell of lithium. Writing the configurations in this way emphasizes the similarity of the configurations of lithium and sodium. Both atoms, which are in the alkali metal family, have only one electron in a valence s subshell outside a filled set of inner shells.
[ce{Li:[He]},2s^1 ce{Na:[Ne]},3s^1]
A chemical reaction results from electron removal, electron addition, or electron sharing of the valence electrons of the different atoms. The path a specific element will take depends on where the electrons are in the atom and how many there are. Thus, it is convenient to separate electrons into two groups. Valence shell electrons (or, more simply, the valence electrons) are the electrons in the highest-numbered shell, or valence shell, while core electrons are the electrons in lower-numbered shells. We can see from the electron configuration of a carbon atom—1s22s22p2—that it has 4 valence electrons (2s22p2) and 2 core electrons (1s2). You will see in the next chapters that the chemical properties of elements are determined by the number of valence electrons.
Example (PageIndex{3})
Examine the electron configuration of neutral phosphorus atoms in Example (PageIndex{1}), 1s22s22p63s23p3 and write the abbreviated notation.
Solution
How Many Phosphorus Valence Electrons
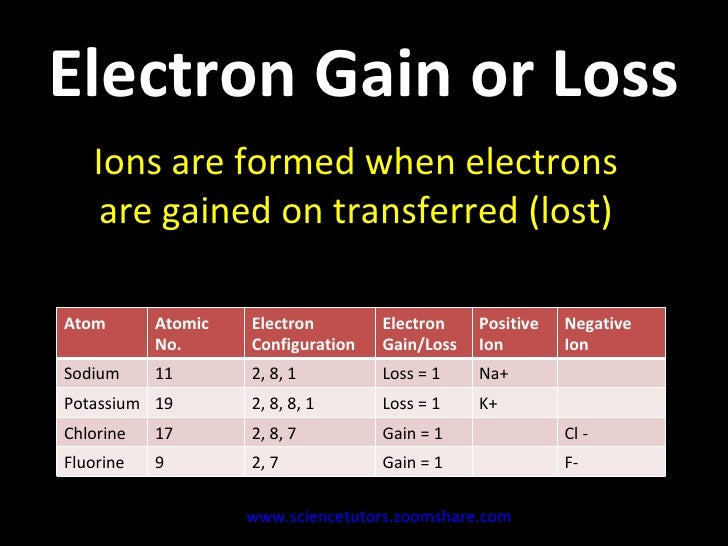
Phosphorus has electron configuration, 1s22s22p63s23p3.
The highest-numbered shell is the third shell (3s23p3): 2 electrons in the 3s subshell and 3 electrons in the 3p subshell. That gives a total of 5 valence electrons.
The 10 inner shell (core) electrons, 1s22s22p6 can be replaced by [Ne] (see Figure (PageIndex{3})). Abbreviated notation is : [Ne]3s23p3
Exercise (PageIndex{3})
Examine the electron configuration of neutral calcium atom (Exercise (PageIndex{2})), 1s22s22p63s23p64s2, and write the abbreviated notation.
The highest-numbered shell is the fourth shell 4s2, which has 2 electrons in the 4s subshell. Hence, Calcium has 2 valence electrons.
The 18 inner-shell (core) electrons, 1s22s22p63s23p6, can be replaced by [Ar], see Figure (PageIndex{3}). The abbreviated notation is: [Ar]4s2
Example (PageIndex{4})
Based on their respective locations in the periodic table (use Figure (PageIndex{3})), determine the number of valence electrons and the valence shell configuration of elements A, B and C.
Solution
Element A is located in Period 2, the 5th position in 2p-block. Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s2) and five valence electrons in 2p (2p5). Answer: 2s22p5. It has 2 + 5 = 7valence electrons.
Element B is located in Period 3, the 2nd position in 3s-block. This means that B has two valence electrons in 3s (3s2). Answer: 3s2.
Element C is located in Period 5, the 1st position in 5s-block). This means that there is only one valence electron in 5s (5s1). Answer: 5s1.
Exercise (PageIndex{4})
Using the location of Na is the periodic table (Figure (PageIndex{3})), draw the shell diagram of sodium atom.
Phosphorus Valence Electrons Number
Sodium (Na) is the first element in the 3rd row (Period 3) in the periodic table. This means that the first shell and second shells of Na atom are filled to the maximum number of electrons.

The first shell (1s) is filled with 2 electrons. The second shell(2s and 2p) has a total of 8 electrons. And, the third (last) shell has1 electron.
The shell diagramof the Na atom is shown below. The shell nearest the nucleus (first shell) has 2 electrons (2 dots), the second shell has 8 electrons and the last (outermost) shell has 1 electron. (2.8.1)
Concept Review Exercises
Phosphorus Valence Electrons And Ion
- How are electrons organized in atoms?
- What information does an electron configuration convey?
- What is the difference between core electrons and valence electrons?
Answers
- Electrons are organized into shells and subshells around nuclei.
- The electron configuration states the arrangement of electrons in shells and subshells.
- Valence electrons are in the highest-numbered shell; all other electrons are core electrons.
Key Takeaway
- Electrons are organized into shells and subshells about the nucleus of an atom.
- The valence electrons determine the reactivity of an atom.
Exercises

